High proton conductivity of NaMg1−xLixHx (PO3)3·yH2O with a three-dimensional open framework in the intermediate temperature range†
Abstract
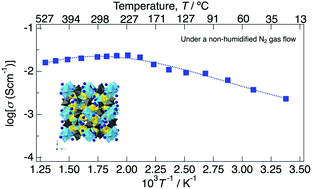
Proton solid electrolytes, which exhibit high proton conductivity and thermal stability in a wide range of intermediate temperatures, are desirable for operating fuel cells at a temperature suitable for applications such as automobiles and cogeneration systems. A new proton conductor NaMg1−xLixHx(PO3)3·yH2O was synthesised by a coprecipitation method. Powder X-ray structure refinement reveals that the three-dimensional tunnel framework is formed by the sharing of corner oxygens of zigzag PO4 tetrahedral chains and face-shared (NaO6)(MgO6) chains. In the three-dimensional tunnel, the oxygen sites of water for crystallisation were observed. In Fourier transform infrared spectroscopy (FTIR) measurement, an increase in absorption peaks corresponding to the modes of O–H and P–O–H bonds for x in NaMg1−xLixHx(PO3)3·yH2O was observed, which reflects the introduction of protons to the crystal structure. Although a multi-step weight loss due to desorption of water of crystallisation was observed for thermogravimetry (TG) curves, NaMg1−xLixHx(PO3)3·yH2O retained the framework up to 800 °C. The proton conductivity shows a positive tendency for x. NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a high proton conductivity of over 10−2 S cm−1 in the temperature range of 150–500 °C, and the value reached 2.4 × 10−2 S cm−1 at 225 °C under a non-humidified atmosphere. The proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O changed with the amount of crystalline water. In a humidified atmosphere (pH2O = 4 kPa), NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a conductivity of over 10−3 S cm−1 from room temperature to 500 °C and reached 2.6 × 10−2 S cm−1 at 225 °C. After keeping the dehydrated sample in the humidified atmosphere at room temperature for 2 h, NaMg0.8Li0.2H0.2(PO3)3·yH2O showed a proton conductivity of over 10−2 S cm−1 again in the intermediate temperature range.


 Please wait while we load your content...
Please wait while we load your content...