Protonation- and electrostatic-interaction-based fluorescence probes for the selective detection of picric acid (2,4,6-trinitrophenol) – an explosive material
Abstract
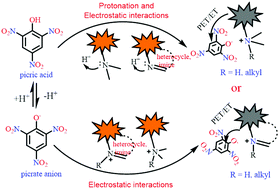
Picric acid, due to its low pKa value, possesses distinct physicochemical features from all other nitroaromatic derivatives, enabling the design of fluorescent probes for its sensitive and selective detection. The electrostatic interactions between the positive charge on the fluorescent probe, which arises due to protonation or the presence of a quaternary nitrogen, and the picrate anion increase the proximity between the fluorescent probe and the analyte, thus increasing the efficiency of the electron or energy transfer processes. This provides a salient feature for the design of fluorescent probes for the selective detection of picric acid over other nitroaromatic compounds. Here, the literature on the use of fluorescent small organic molecules for the selective detection of picric acid based on electrostatic interactions between positively charged/protonated fluorophores and their ability to selectively detect picric acid has been discussed. The fluorescent probes have been classified into three broad categories – neutral fluorophores, which are selectively protonated by picric acid; positively charged probes, which exhibit electrostatic interactions with the picrate anion; and metal complexes with protonatable sites. This comprehensive review will prove a benchmark for the future design of fluorescent probes for picric acid.


 Please wait while we load your content...
Please wait while we load your content...