Simultaneous improvement of kinetics and thermodynamics based on SrF2 and SrF2@Gr additives on hydrogen sorption in MgH2†
Abstract
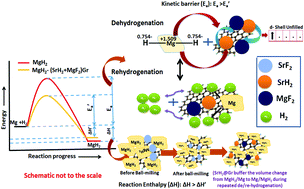
Herein we describe and discuss the effect of significant advantages of the alkaline earth fluoride additive SrF2 on the improvement of the kinetics, thermodynamics, and cyclability of the frontier hydrogen storage material MgH2. Strontium fluoride, SrF2, which has been used as an additive for the first time in the present study, has an elemental electronegativity difference of 3.04 (the highest amongst the fluorides) as compared to 2.38 for NbF5, one of the best-known catalysts so far for MgH2. Therefore, SrF2 is highly ionic and will readily react with MgH2, which is not only ionic but also has a polar covalent character. SrF2 reacts with MgH2 to yield magnesium fluoride (MgF2) and strontium hydride (SrH2) which act as catalysts. The present investigations have revealed that both (MgF2 + SrH2) and their graphene templated version, (MgF2 + SrH2)@Gr, work as better catalysts for MgH2 than NbF5. Thus the desorption of H2 from ball-milled MgH2 catalyzed by (MgF2 + SrH2) corresponds to 5.30 wt% in 15 min, and for (MgF2 + SrH2)@Gr the desorption is 6.01 wt% in 15 min at 290 °C. Also, the onset desorption temperatures for MgH2 with (MgF2 + SrH2) and (MgF2 + SrH2)@Gr catalysts are 261 °C and 231 °C, respectively, which are 95 °C and 125 °C lower than that for ball-milled MgH2. The H2 absorption for (MgF2 + SrH2) catalyzed MgH2 is found to be 6.00 wt% in 5 min at 290 °C. For (MgF2 + SrH2)@Gr catalyzed MgH2 the hydrogen absorption is 6.16 wt% in 2 min at 290 °C. The change in desorption enthalpy for MgH2–(MgF2 + SrH2)@Gr is 67.60 kJ mol−1 as compared to 74.84 kJ mol−1 for MgH2–(MgF2 + SrH2). The storage capacity for MgH2–(MgF2 + SrH2)@Gr remains ∼6.00 wt% even after 15 cycles, which corresponds to excellent cyclability. A feasible catalytic mechanism arising from (MgF2 + SrH2) and (MgF2 + SrH2)@Gr catalysts on hydrogen sorption in MgH2 has been proposed based on X-ray diffraction, Raman spectroscopy, Fourier transmission infrared spectroscopy, and transmission/scanning electron microscopic studies. The present study is the first of its type where absorption/desorption kinetics, thermodynamics, and cyclability for MgH2 have all been improved by the use of the single additive SrF2 and the derived catalyst MgF2 + SrH2.


 Please wait while we load your content...
Please wait while we load your content...