Experimental validation of high electrical conductivity in Ni-rich LaNi1−xFexO3 solid solutions (x ≤ 0.4) in high-temperature oxidizing atmospheres†
Abstract
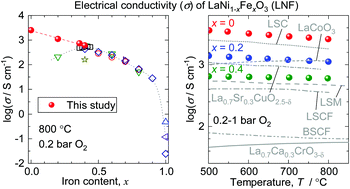
LaNi1−xFexO3 solid solutions are an interesting system exhibiting a composition-controlled metal–insulator transition and are also potential cathode materials for solid oxide fuel cells, but the composition dependence of their electrical conductivity is still an open question due to the difficulty in synthesis and sintering. Here, in contrast to previous studies, it is demonstrated that the electrical conductivity of LaNi1−xFexO3 monotonically increases with decreasing x (increasing Ni content), reaching as high as 1.0 × 104 S cm−1 at room temperature and 2.5 × 103 S cm−1 at 800 °C in 0.2 bar O2 when x = 0. The accurate electrical conductivity measurements of LaNi1−xFexO3 with high Ni contents (0 ≤ x ≤ 0.4) are realized using fully dense single-phase polycrystalline samples prepared by the post-sintering oxidation process. The results suggest that LaNi1−xFexO3 with a higher Ni content might be more suitable as the cathodes than the widely-studied composition LaNi0.6Fe0.4O3. Furthermore, LaNiO3 is now considered to have the highest electrical conductivity among precious-metal-free oxides in high-temperature oxidizing atmospheres and can find more applications.


 Please wait while we load your content...
Please wait while we load your content...