Ammonia synthesis using Fe/BZY–RuO2 catalysts and a caesium dihydrogen phosphate-based electrolyte at intermediate temperatures†
Abstract
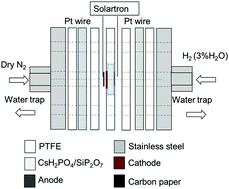
In this study, we developed an Fe2O3/BZY (yttrium-doped barium zirconate)–RuO2 (Fe/BZY–RuO2) cathode catalyst, which was applied to the electrochemical synthesis of NH3 using a proton-conducting electrolyte, CsH2PO4/SiP2O7, at 220 °C and ambient pressure. The highest faradaic efficiency of 7.1% was achieved at −0.4 V (vs. open-circuit voltage (OCV)) and the highest NH3 yield rate of 4.5 × 10−10 mol (s cm2)−1 was achieved at −1.5 V (vs. OCV). We also successfully detected N2H4 and NH3 at −0.2 V (vs. OCV), which indicated that the N2 reduction proceeded via an associative mechanism. A potentiostatic pulse experiment was conducted under a feed of Ar or N2 in the cathode at different applied voltages to investigate the N2 reduction reaction (NRR) mechanism. A model was developed to fit the current response of the potentiostatic pulse experiment, which comprised the decomposition of adsorbed intermediates on the surface of the cathode catalyst, diffusion of H in the cathode catalyst, and an electrical double layer. The results revealed that the rate constant estimated by the model for the decomposition of intermediates, such as NH or N2H, was lowest at −0.2 V, where N2H4 was detected. The fitting results also indicated that the NRR proceeded via an associative mechanism at lower applied voltages, while a dissociative mechanism dominated at higher applied voltages.


 Please wait while we load your content...
Please wait while we load your content...