Effect of different parameters on caustic magnesia hydration and magnesium hydroxide rheology: a review
Abstract
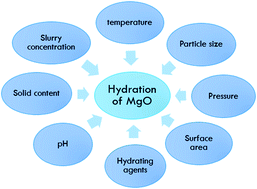
Magnesium oxide and magnesium hydroxide are two compounds that show favorable properties, leading to their use in many industrial applications. This review discusses the methods of synthesizing magnesium oxide and magnesium hydroxide from dolomite, magnesite, brines and sea water using the conventional methods of precipitation and hydration. Nanoparticle preparation is via precipitation using sonochemical, sol–gel and electrochemical methods or via hydration using solvothermal and hydrothermal methods. Moreover, the application of both magnesium oxide and magnesium hydroxide is discussed in this review. This review article also provides extensive information on the hydration process of magnesium oxide and the effect of different parameters including the pH, temperature, surface area, particle size, hydrating agents, etc., which affect the rheological behavior of Mg(OH)2. This review is a source of scientific as well as practical information on the applications of Mg(OH)2 and MgO at the present time and on the methods of obtaining them, in the light of available and applied research techniques and discoveries made so far in the field of advanced powder technology.


 Please wait while we load your content...
Please wait while we load your content...