3D magnetically controlled spatiotemporal probing and actuation of collagen networks from a single cell perspective†
Abstract
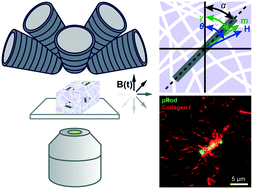
Cells continuously sense and react to mechanical cues from their surrounding matrix, which consists of a fibrous network of biopolymers that influences their fate and behavior. Several powerful methods employing magnetic control have been developed to assess the micromechanical properties within extracellular matrix (ECM) models hosting cells. However, many of these are limited to in-plane sensing and actuation, which does not allow the matrix to be probed within its full 3D context. Moreover, little attention has been given to factors specific to the model ECM systems that can profoundly influence the cells contained there. Here we present methods to spatiotemporally probe and manipulate extracellular matrix networks at the scale relevant to cells using magnetic microprobes (μRods). Our techniques leverage 3D magnetic field generation, physical modeling, and image analysis to examine and apply mechanical stimuli to fibrous collagen matrices. We determined shear moduli ranging between hundreds of Pa to tens of kPa and modeled the effects of proximity to rigid surfaces and local fiber densification. We analyzed the spatial extent and dynamics of matrix deformation produced in response to magnetic torques on the order of 10 pNm, deflecting fibers over an area spanning tens of micrometers. Finally, we demonstrate 3D actuation and pose extraction of fluorescently labelled μRods.


 Please wait while we load your content...
Please wait while we load your content...