Thermoplastic nanofluidic devices for identifying abasic sites in single DNA molecules†
Abstract
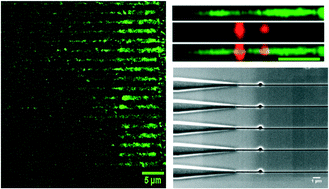
DNA damage can take many forms such as double-strand breaks and/or the formation of abasic (apurinic/apyrimidinic; AP) sites. The presence of AP sites can be used to determine therapeutic efficacy of many drugs, such as doxorubicin. While there are different assays to search for DNA damage, they are fraught with limitations, such as the need for large amounts of DNA secured from millions of cells. This is challenging due to the growing importance of using liquid biopsies as a source of biomarkers for many in vitro diagnostic assays. To accommodate the mass limits imposed by the use of liquid biopsies, we report a single-molecule DNA damage assay that uses plastic nanofluidic chips to stretch DNA to near its full contour length when the channel dimensions (width and depth) are near the persistence length (∼50 nm) of double-stranded (ds) DNA. The nanofluidic chip consisted of input funnels for high loading efficiency of single DNA molecules, entropic traps to store the DNA and simultaneously load a series of nanochannels for high throughput processing, and an array of stretching nanochannels to read the AP sites. Single dsDNA molecules, which were labeled with an intercalating dye and a biotinylated aldehyde reactive probe (bARP), could be parked in the stretching nanochannels, where the AP sites were read directly using a dual-color fluorescence microscope equipped with an EMCCD camera. One color of the microscope was used to read the DNA length and the second color detected the AP sites. The nanofluidic chip was made from thermoplastics via nanoimprint lithography, which obviated the need for direct writing the devices in glass or quartz using focused ion beam milling. We show that we can read the frequency of AP sites in single dsDNA molecules with the frequency of AP sites determined by associating fluorescently-labeled streptavidin with bARP through a biotin/streptavidin complex.


 Please wait while we load your content...
Please wait while we load your content...