Development of a sample preparation method for accurate analysis of 24 elemental impurities in oral drug products by ICP-MS according to USP/ICH guidelines
Abstract
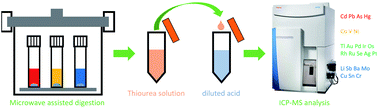
Regulatory requirements for the analysis of 24 elemental impurities (Li, V, Cr, Co, Ni, Cu, As, Se, Mo, Ru, Rh, Pd, Ag, Cd, Sn, Sb, Ba, Os, Ir, Pt, Au, Hg, Tl and Pb) within pharmaceutical products are now included in the guidelines of the USP and ICH. Accurately screening all 24 elements in a single analysis is a challenging task due to different physico-chemical properties of the analytes and diverse compositions of available pharmaceuticals. In the present work, a new sample preparation method was developed for accurate analysis of all 24 USP target elements in five oral drug products (terbutaline sulfate tablets, sertraline hydrochloride tablets, cilostazol tablets, rosuvastatin calcium tablets and sitaglipin phosphate/metformin hydrochloride tablets). Method development particularly involved the stabilization and reliable determination of Os, Se and Au. Sample preparation was performed by microwave-assisted acid digestion using aqua regia followed by a two-step dilution procedure. The digest was first diluted with a simple thiourea solution in order to stabilize Os, and then an aliquot was diluted with a diluted acid solution to eliminate the matrix effect of Se and Au before quantitation by ICP-MS. The influences of different digestion solutions and different post-digestion diluents were investigated. The ICP-MS methodology covering the determination of all 24 elements was successfully validated in terms of linearity, accuracy, precision, the LOD and the LOQ. It is expected that the proposed method can be implemented for routine analysis of a broad spectrum of oral drug products, and help official medicine control laboratories in the quality assessment of drug products in the market.


 Please wait while we load your content...
Please wait while we load your content...