Divergent Ritter-type amination via photoredox catalytic four-component radical-polar crossover reactions†
Abstract
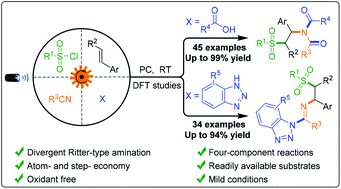
A green and practical divergent Ritter-type amination via a photoredox catalytic four-component radical-polar crossover process was developed. This versatile protocol presents a modular and powerful strategy to access functionalized β-sulfonyl imides and imines under mild conditions from readily available and stable substrates with high atom-, step- and redox economy. We manipulated carboxylic acids and benzotriazoles to precisely attack the nitrilium ion instead of the carbocation, thus preventing many complicated side reactions of the photocatalytic reactions. Furthermore, the synthetic utility of this divergent Ritter reaction is further demonstrated by the late-stage modification of pharmaceutical and natural product derivatives.


 Please wait while we load your content...
Please wait while we load your content...