An iron(iii)-catalyzed dehydrogenative cross-coupling reaction of indoles with benzylamines to prepare 3-aminoindole derivatives†
Abstract
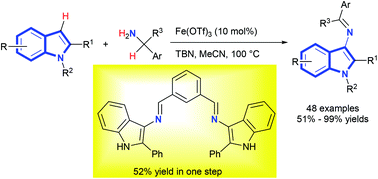
We report a green cascade approach to prepare a variety of 3-aminoindole derivatives in good to excellent yields through an iron(III)-catalyzed dehydrogenative cross-coupling reaction of 2-arylindoles and primary benzylamines under mild reaction conditions. Mechanistic studies show that a cascade reaction involves a tert-butyl nitrite (TBN)-mediated nitrosation of 2-substituted indoles and a 1,5-hydrogen shift to afford indolenine oximes, sequential iron(III)-catalyzed condensation and a 1,5-hydrogen shift over four steps in a one-pot reaction. The reaction shows a broad substrate scope of indoles and benzylamines and tolerates a wide range of functional groups. Moreover, the reaction is easily performed at the gram scale without producing waste after the reaction is completed. The 3-aminoindole product is purified by simple extraction, washing, and recrystallization without flash column chromatography. A double imine ligand containing the 3-aminoindole unit is facile to obtain in a 52% yield in one step. The present method highlights readily available starting materials, a simple purification procedure, and the usage of cheap, nontoxic, and environmentally benign iron(III) catalysts.


 Please wait while we load your content...
Please wait while we load your content...