A chemo-enzymatic oxidation/aldol sequential process directly converts arylbenzyl alcohols and cyclohexanol into chiral β-hydroxy carbonyls†
Abstract
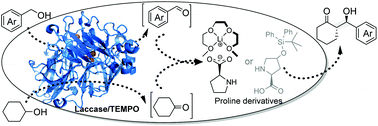
The development of a combination enzyme and organocatalyst for aqueous sequential organic transformation has great significance, in that it is not only environmentally friendly but also overcomes only a single methodological drawback, either in the chemical or biological process. Herein, through the utilization of the bulky steric hindrance of chiral proline derivatives, an integrated laccase and proline as a chemo-enzymatic co-catalyst system is developed. It enables an efficient oxidation/aldol enantioselective sequential reaction to be accomplished, overcoming the mutual deactivation issue. As we present in this study, this one-pot organic transformation, an initial laccase-mediated oxidation of arylbenzyl alcohols and cyclohexanol to form aldehydes and cyclohexanone, followed by a subsequent proline derivative-catalyzed aldol condensation of the in situ generated intermediates, provides various 1,2-diastereoisomeric chiral β-hydroxy ketones with acceptable yields and high enantio-/diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...