Metal-free hydrogen evolution cross-coupling enabled by synergistic photoredox and polarity reversal catalysis†
Abstract
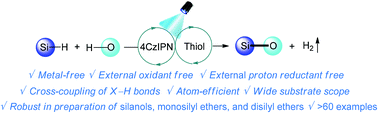
A synergistic combination of photoredox and polarity reversal catalysis enabled a hydrogen evolution cross-coupling of silanes with H2O, alcohols, phenols, and silanols, which afforded the corresponding silanols, monosilyl ethers, and disilyl ethers, respectively, in moderate to excellent yields. The dehydrogenative cross-coupling of Si–H and O–H proceeded smoothly with broad substrate scope and good functional group compatibility in the presence of only an organophotocatalyst 4-CzIPN and a thiol HAT catalyst, without the requirement of any metals, external oxidants and proton reductants, which is distinct from the previously reported photocatalytic hydrogen evolution cross-coupling reactions where a proton reduction cocatalyst such as a cobalt complex is generally required. Mechanistically, a silyl cation intermediate is generated to facilitate the cross-coupling reaction, which therefore represents an unprecedented approach for the generation of silyl cation via visible-light photoredox catalysis.


 Please wait while we load your content...
Please wait while we load your content...