Bifunctionalization of unsaturated bonds via carboxylative cyclization with CO2: a sustainable access to heterocyclic compounds
Abstract
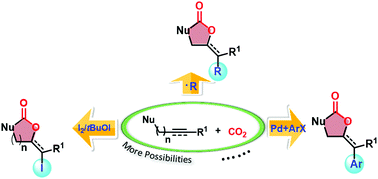
CO2 valorization into valuable chemicals has attracted much attention, among which carboxylative cyclization represents an appealing transformation strategy to synthesize heterocyclic compounds. Conventionally, the carboxylative cyclization comprises CO2 capture and subsequent nucleophilic attack of the resulting carboxylic anion to an unsaturated bond, by which the monofunctionalization of the unsaturated bond is realized and the heterocyclic compounds, such as 5-/6-membered cyclolactone, cyclocarbonate, and oxazolidinone, are accordingly generated. Nowadays, the CO2-participated multicomponent carboxylative cyclizations have evoked intense interest due to their ability to perform the bifunctionalization of the unsaturated bond in the ring-closing step, thus assembling complex molecules. Up to now, by activating the unsaturated bonds with functionalized reagents and designing cascade reactions of organometallic compounds, substituents including iodine, trifluoromethyl, perfluoroalkyl, alkyl, cyanoalkyl, allyl, aryl, phenoxy, allenyl, selenyl, amino, and phosphonyl groups have been incorporated into the cyclocarbonate or oxazolidinone compounds successfully through the multicomponent carboxylative cyclizations. Given the tremendous utility of this synthetic strategy in heterocyclic compound synthesis, this review summarizes the progress of multicomponent carboxylative cyclizations to spur the further development of this field.
- This article is part of the themed collection: Green Chemistry Reviews


 Please wait while we load your content...
Please wait while we load your content...