Facile growth of transition metal hydroxide nanosheets on porous nickel foam for efficient electrooxidation of benzyl alcohol†
Abstract
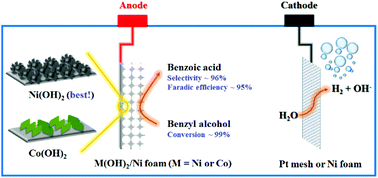
Porous transition metal hydroxide (M(OH)2, M = Ni or Co) nanosheet/Ni foam electrodes were prepared by a facile electrodeposition method. The catalytic activities of the as-prepared porous electrodes for the electrooxidation of benzyl alcohol were investigated in NaOH aqueous solution. It was found that the Ni(OH)2 nanosheet/Ni foam electrode exhibited a superior current density to the obtained Co(OH)2 nanosheet/Ni foam electrode for the electrooxidation of benzyl alcohol, in which the Ni(OH)2 nanosheet electrode required an extremely low potential of ∼1.33 V vs. RHE to achieve a current density of 100 mA cm−2. The results of the durability test revealed that it exhibited an outstanding stability for the electrooxidation of benzyl alcohol. Moreover, the electrooxidation of benzyl alcohol for the green synthesis of benzoic acid with the simultaneous generation of hydrogen could be easily implemented in a two-electrode configuration by using noble-metal-free catalysts. When benzyl alcohol was fully oxidized, the optimized selectivity of benzoic acid and the faradaic efficiency for the electrooxidation of BnOH could reach 96% and 95%, respectively.


 Please wait while we load your content...
Please wait while we load your content...