Synergistic recycling and conversion of spent Li-ion battery leachate into highly efficient oxygen evolution catalysts†
Abstract
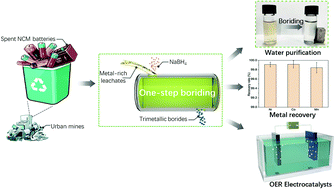
Converting spent Li-ion batteries (LIBs) into highly efficient energy conversion catalysts in a facile manner is a win-win strategy in addressing the metal resources shortage and clean energy problems. Herein, we employed a one-pot boriding strategy to turn the spent LiNiCoMnO2 batteries into magnetic mixed metallic borides (NiCoMnBs) for efficient oxygen evolution reaction (OER). A metal ion recovery rate of 99.91%, 99.92%, and 99.84% has been achieved for Ni, Co and Mn, respectively. The optimized catalyst demonstrates a highly competitive OER activity, and only an overpotential of 372 mV is required to drive the high current density of 500 mA cm−2, surpassing many other Ni, Co, Mn mono-, bi- or tri-metallic OER catalysts as well as the commercial RuO2 catalyst. Detailed mechanism studies suggest that the superior OER performance is mainly ascribed to B etching induced in situ generation of the core@shell structured tri-metal borides@(oxy)hydroxides nanostructures during the water oxidation process. Fast charge-transfer kinetics and the hierarchical amorphous/nanocrystalline structure with rich catalytic active sites are also aiding the high activity. The boriding strategy reported here can be further applied to synergistically recycle and regenerate efficient electroactive materials from other metal-rich wastes for achieving a sustainable energy future.


 Please wait while we load your content...
Please wait while we load your content...