BF3·Et2O as a metal-free catalyst for direct reductive amination of aldehydes with amines using formic acid as a reductant†
Abstract
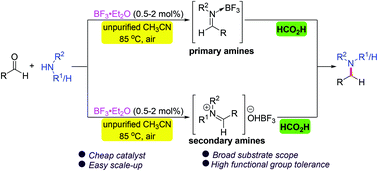
A versatile metal- and base-free direct reductive amination of aldehydes with amines using formic acid as a reductant under the catalysis of inexpensive BF3·Et2O has been developed. A wide range of primary and secondary amines and diversely substituted aldehydes are compatible with this transformation, allowing facile access to various secondary and tertiary amines in high yields with wide functional group tolerance. Moreover, the method is convenient for the late-stage functionalization of bioactive compounds and preparation of commercialized drug molecules and biologically relevant N-heterocycles. The procedure has the advantages of simple operation and workup and easy scale-up, and does not require dry conditions, an inert atmosphere or a water scavenger. Mechanistic studies reveal the involvement of imine activation by BF3 and hydride transfer from formic acid.


 Please wait while we load your content...
Please wait while we load your content...