Photocatalyst- and additive-free site-specific C(sp3)–H hydrazination of glycine derivatives and peptides†
Abstract
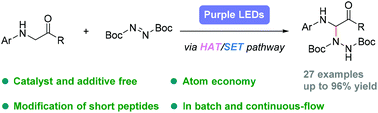
A visible light-mediated protocol for site-specific C(sp3)–H hydrazination of glycine derivatives and peptides with azo compounds has been developed. This C(sp3)–N coupling reaction proceeded smoothly under photo-irradiation conditions in the absence of any catalysts and additives, thus providing a convenient and facile method for efficient preparation of unnatural α-amino acids and precise modification of short peptides. Mechanism investigations revealed that single electron transfer (SET) and hydrogen atom transfer (HAT) may occur during this transformation.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...