Visible light-induced recyclable g-C3N4 catalyzed thiocyanation of C(sp2)–H bonds in sustainable solvents†‡
Abstract
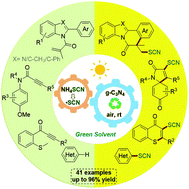
A metal-free photocatalytic strategy for the preparation of thiocyanated heterocycles from inexpensive NH4SCN has been developed using carbon nitride (g-C3N4) as a general heterogeneous catalyst in a green solvent under an air atmosphere and the irradiation of a blue LED. Various thiocyanated heterocycles including indolo[2,1-a]isoquinolin-6(5H)-ones, benzimidazo[2,1-a]isoquinolin-6(5H)-ones, thioflavones, azaspiro[4.5]trienones and imidazo[1,2-a]pyridines were successfully synthesized in good yields (up to 96%). Importantly, this metal-free and external-oxidant-free procedure employed dimethyl carbonate as a green medium, avoiding the traditional volatile organic solvents. Moreover, the recyclable g-C3N4 catalyst could be used at least 8 times without significant loss of activities.


 Please wait while we load your content...
Please wait while we load your content...