Oxo-functionalised mesoionic NHC nickel complexes for selective electrocatalytic reduction of CO2 to formate†
Abstract
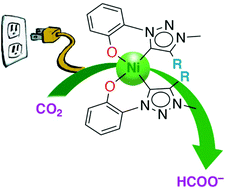
Strategies for the conversion of CO2 to valuable products are paramount for reducing the environmental risks associated with high levels of this greenhouse gas and offer unique opportunities for transforming waste into useful products. While catalysts based on nickel as an Earth-abundant metal for the sustainable reduction of CO2 are known, the vast majority produce predominantly CO as a product. Here, efficient and selective CO2 reduction to formate as a synthetically valuable product has been accomplished with novel nickel complexes containing a tailored C,O-bidentate chelating mesoionic carbene ligand. These nickel(II) complexes are easily accessible and show excellent catalytic activity for electrochemical H+ reduction to H2 (from HOAc in MeCN), and CO2 reduction (from CO2-saturated MeOH/MeCN solution) with high faradaic efficiency to yield formate exclusively as an industrially and synthetically valuable product from CO2. The most active catalyst precursor features the 4,6-di-tert-butyl substituted phenolate triazolylidene ligand, tolerates different proton donors including water, and reaches an unprecedented faradaic efficiency of 83% for formate production, constituting the most active and selective Ni-based system known to date for converting CO2 into formate as an important commodity chemical.


 Please wait while we load your content...
Please wait while we load your content...