Construction and optimization of a microbial platform for sustainable biosynthesis of poly-N-acetyllactosamine glycoprotein in the cytoplasm for detecting tumor biomarker galectin-3†
Abstract
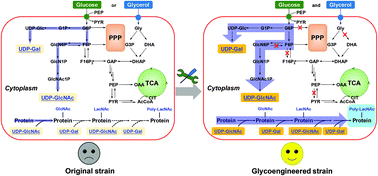
Galectin-3 (Gal-3), a member of the β-galactoside-binding lectin family, involved in tumor angiogenesis and metastasis, is considered a diagnostic biomarker and a promising treatment target for early cancer. Gal-3 recognizes the poly-N-acetyllactosamine (poly-LacNAc)-based carbohydrate motifs of glycoproteins and glycolipids. To date, protein glycosylation with poly-LacNAc has been mainly achieved by chemical or chemoenzymatic strategies. In this study, a green artificial biosynthesis pathway for catalyzing the poly-LacNAc glycosylation of protein was designed and developed in the cytoplasm of Escherichia coli. The pathway enlists a cytoplasmic sequential O-glycosylation polypeptide-glycosyltransferase from Streptococcus parasanguinis to establish a site-specific primer N-acetylglucosamine on the target protein, and two glycosyltransferases from Neisseria meningitidis and Helicobacter pylori to extend the poly-LacNAc chain. Rewiring the metabolic network of the strain, by applying a carbon absorption enforced intersection and orthogonal utilization system, achieved the goal of poly-LacNAc glycoprotein biosynthesis in the cytoplasm. This approach coupled phosphoenolpyruvate (PEP) generation from the second carbon source, glycerol, with PEP consumption by the PEP-dependent phosphotransferase system, to facilitate noncatabolic utilization of glucose. The content of LacNAc-linked and poly-LacNAc-linked glycoprotein purified from the engineered strain reached 17 mg L−1, ∼10 times that purified from the starting strain. Furthermore, the degree of polymerization of the poly-LacNAc glycoprotein was further improved by optimizing the pathway flux toward the availability of the two key nucleotide sugars. The purified glycoprotein carrying the LacNAc epitope displayed a high affinity for Gal-3. Finally, the concentration of poly-LacNAc glycoprotein reached 45.7 mg L−1 through optimizing various culture conditions. The cytoplasmic glycoengineering strategies used in this study open up new avenues for the efficient and green manufacturing of various glycoprotein structures with diverse biomedical applications, thus reducing or eliminating the use or generation of hazardous substances.


 Please wait while we load your content...
Please wait while we load your content...