Catalytic intermolecular aldol reactions of transient amide enolates in domino Michael/aldol reactions of nitroalkanes, acrylamides, and aldehydes†
Abstract
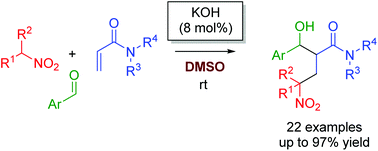
Three-component reactions of nitroalkanes, acrylamides, and aldehydes by using a catalytic amount of KOH in DMSO gave β′-hydroxy-γ-nitro amides. Mechanistic studies suggested that the reactions proceeded by domino Michael/aldol reactions. The highly nucleophilic nature of transient amide enolates that were formed by Michael addition of nitronate anions to acrylamides in DMSO enabled the realization of catalytic intermolecular aldol reactions of amide enolates in the presence of acidic nitroalkanes.


 Please wait while we load your content...
Please wait while we load your content...