Copper-catalysed synthesis of α-alkylidene cyclic carbonates from propargylic alcohols and CO2†
Abstract
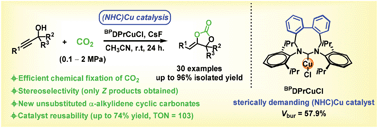
We report a N-heterocyclic carbene copper(I) complex-catalysed formal cycloaddition between readily available propargylic alcohols and carbon dioxide at room temperature. By using the combination of a sterically demanding BPDPrCuCl complex (BPDPr = 1,3-bis(2,6-diisopropylphenyl)-1,3-diazonine-2-ylidene) and CsF, as catalytic system, primary propargylic alcohols are efficiently converted to the corresponding α-alkylidene cyclic carbonates. Gram scale (up to 89% yield) and reusability experiments (74% global yield, turnover number value = 103) showcase the robustness of the catalytic system. This practically simple protocol also tolerates secondary and tertiary propargylic alcohols under CO2 at atmospheric pressure, enabling the direct synthesis of substituted and unsubstituted α-alkylidene cyclic carbonates at room temperature.


 Please wait while we load your content...
Please wait while we load your content...