Photo-triggered self-catalyzed fluoroalkylation/cyclization of unactivated alkenes: synthesis of quinazolinones containing the CF2R group†
Abstract
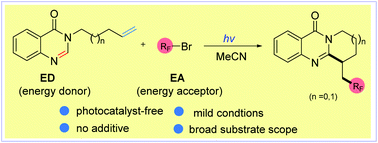
A novel photo-triggered self-catalyzed fluoroalkylation/cyclization of quinazolinones containing unactivated alkenes with various fluoroalkyl bromides has been developed. This transformation exhibits excellent substrate generality with respect to both the coupling partners. Of note is that this is the first example describing the Csp3–Br bond homolysis of alkyl bromides via a substrate (quinazolinones) induced energy transfer process. Additionally, the mild conditions, tolerance to a wide range of functional groups and operational simplicity make this protocol practical for the synthesis of fluorine-containing ring-fused quinazolinones.


 Please wait while we load your content...
Please wait while we load your content...