Thio-assisted reductive electrolytic cleavage of lignin β-O-4 models and authentic lignin†
Abstract
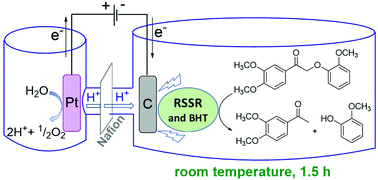
Avoiding the use of expensive catalysts and harsh conditions such as elevated temperatures and high pressures is a critical goal in lignin depolymerization and valorization. In this study, we present a thio-assisted electrocatalytic reductive approach using inexpensive reticulated vitreous carbon (RVC) as the working cathode to cleave the β-O-4-type linkages in keto aryl ethers. In the presence of a pre-electrolyzed disulfide (2,2′-dithiodiethanol) and a radical inhibitor (BHT) at room temperature at a current density of 2.5 mA cm−2, cathodic reduction of nonphenolic β-O-4 dimers afforded over 90% of the corresponding monomeric C–O cleavage products in only 1.5 h. Extended to DDQ-oxidized poplar lignin, this combination of electric current and disulfide, applied over 6 h, released 36 wt% of ethyl acetate soluble fragments and 26 wt% of aqueous soluble fragments, leaving only 38 wt% of insoluble residue. These findings represent a significant improvement over the current alone values (24 wt% ethyl acetate soluble; 22 wt% aqueous soluble; 54 wt% insoluble residue) and represent an important next step in our efforts to develop a mild electrochemical method for reductive lignin deconstruction.


 Please wait while we load your content...
Please wait while we load your content...