Visible light-mediated metal-free double bond deuteration of substituted phenylalkenes†
Abstract
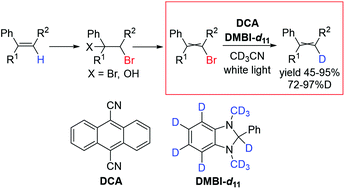
Various bromophenylalkenes were reductively photodebrominated by using 1,3-dimethyl-2-phenyl-1H-benzo-[d]imidazoline (DMBI) and 9,10-dicyanoanthracene. With deuterated DMBI analogs (the most effective was DMBI-d11), satisfactory to excellent isotopic yields were obtained. DMBI-d11 could also be regenerated from the reaction mixtures with a recovery rate of up to 50%. The combination of the photodebromination reaction with conventional methods for bromoalkene synthesis enables sequential monodeuteration of a double bond without the necessity of a metal catalyst.


 Please wait while we load your content...
Please wait while we load your content...