Antihypertensive potential of fermented milk: the contribution of lactic acid bacteria proteolysis system and the resultant angiotensin-converting enzyme inhibitory peptide
Abstract
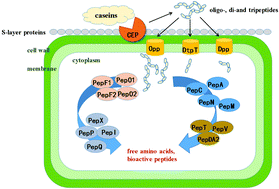
Hypertension has become an increasing health concern given that it is a major risk for cardiovascular disease. Synthetic antihypertensive drugs, including angiotensin-converting enzyme (ACE) inhibitors, effectively control high blood pressure but are associated with unpleasant side effects. Milk fermented by certain lactic acid bacteria (LAB) provides energetic contributions to the management of hypertension, especially the regulation of ACE. LAB are important food-grade microbial organisms that release ACE inhibitory peptides through their unique proteolysis system, which consists of cell-envelope proteinases (CEPs), transporter systems, and intracellular peptidases. Thus, the description of LAB proteolysis system genes and their contributions to ACE inhibitory peptide production is a challenging but promising study. This review provides a survey of LABs with potential ACE inhibitory activity and investigates the research progress of LAB proteolytic systems with an emphasis on the correlation of their components and ACE inhibitory activity. Subsequently, a depiction of the ACE inhibitory peptide action mechanism, structure–activity relationship and bioavailability is presented. The improved functional annotation of LAB proteolytic system genes will provide an excellent framework for future experimental validations of predicted ACE inhibitory activity in fermented milk.


 Please wait while we load your content...
Please wait while we load your content...