The necessity of enzymatically hydrolyzing walnut protein to exert antihypertensive activity based on in vitro simulated digestion and in vivo verification
Abstract
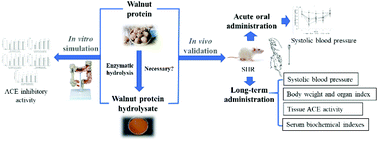
Since not all proteins are suitable for preparing bioactive peptides by enzymatic degradation, the purpose of this study is to evaluate the necessity of walnut protein (WP) enzymolysis to exert its potential antihypertensive activity. Five proteases were used to hydrolyze WP to produce WP hydrolysate (WPH) enzymatically. The angiotensin-I-converting enzyme (ACE) inhibitory activity of WP and WPH before and after simulated digestion in vitro was measured, and the antihypertensive effect was evaluated in vivo. The results showed that after simulated digestion in vitro, the ACE inhibitory activity of WP digests (44.85%) was not significantly different from that of WPH digests (p > 0.05). In vivo experimental results showed that both WP and WPH had significant blood pressure lowering effects in the acute and long-term administrative experiments. The mechanism of its antihypertensive activities was regulating the balance of the renin–angiotensin–aldosterone system and the kallikrein–kinin system by inhibiting ACE activities in tissues and regulating the level of endothelium-derived vasoconstrictor factors and relaxing factors in serum. It seems unnecessary to carry out enzymatic hydrolysis to produce walnut peptides with antihypertensive activity.


 Please wait while we load your content...
Please wait while we load your content...