Changes in the secondary structures and zeta potential of soybean peptide and its calcium complexes in different solution environments
Abstract
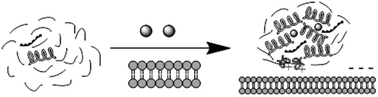
To illustrate the relationship between environment hydrophobicity and soybean peptide and its calcium complexes when they are absorbed transmembrane, different solution environments (HBS buffer, TFE hydrophobic solution and cell suspension) were used to simulate hydrophilic and hydrophobic environments. In this study, soybean peptides (10–30 kDa) with a high calcium binding capacity were prepared by enzymatic hydrolysis and ultrafiltration. The results of cell experiments showed that the peptide could transport calcium into cells for absorption. Secondary structure changes of the peptide and its calcium complexes in different solution environments showed that the secondary structure of the peptide changed during the transmembrane absorption, and the contents of α-helix and β-sheet structures increased. Besides, the β-sheet structures in the peptide–calcium complexes were further converted to an α-helix structure. This conversion may be induced by the hydrophobicity of peptide solutions. In addition, when the conformation changes, the positively charged peptides in the sample will be exposed and then interact with cells, which is beneficial for the transmembrane of peptide–calcium complexes.


 Please wait while we load your content...
Please wait while we load your content...