A tale of two metal ions: contrasting behaviors of high oxidation states of Cu and Mn in a bicarbonate–H2O2 system†
Abstract
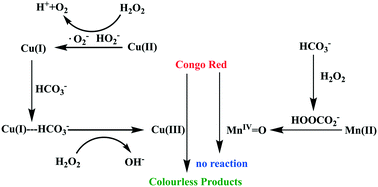
In this study, the contrasting activities and pathways of Mn2+/Cu2+–HCO3− systems for Congo red (CR) dye degradation are presented. The Mn2+–HCO3− system (with the well-known MnIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O as the reactive species) did not degrade CR, while the Cu2+–HCO3− system (with high-valent Cu proposed as the reactive species) had a good oxidizing ability for CR. Further, HO radicals in the classic Fenton-like system made no contribution to CR degradation with both Cu and Fe ions as catalysts. The results indicate that reactive species in metal–HCO3− AOPs need prudent examination and better understanding of the underlying mechanism would benefit the development of more efficient AOPs.
O as the reactive species) did not degrade CR, while the Cu2+–HCO3− system (with high-valent Cu proposed as the reactive species) had a good oxidizing ability for CR. Further, HO radicals in the classic Fenton-like system made no contribution to CR degradation with both Cu and Fe ions as catalysts. The results indicate that reactive species in metal–HCO3− AOPs need prudent examination and better understanding of the underlying mechanism would benefit the development of more efficient AOPs.


 Please wait while we load your content...
Please wait while we load your content...