Albumin protein coronas render nanoparticles surface active: consonant interactions at air–water and at lipid monolayer interfaces†
Abstract
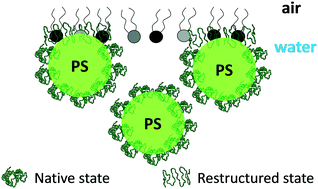
Protein coronas are known to alter the physicochemical properties, colloidal stability, and biological fate of nanoparticles. Using human serum albumin (HSA) and polystyrene nanoparticles (NPs) with anionic or cationic surface chemistries, we show that protein coronas also govern the surface activity of PS nanoparticles as well as their interactions with a model red blood cell (RBC) lipid monolayer. The adsorption kinetics of bare nanoparticles (no corona) and nanoparticles with a hard corona (HC) at an air–water interface were well-described theoretically, which revealed that the adsorption energy was greater with the corona due to hydrophobic interactions that were enhanced with protein restructuring. Corona complexation increased the concentration of nanoparticles at the interface and led to the formation of interfacial aggregates. Despite clear differences in monolayer structure, the compressibility of PS–HC monolayers was similar to free HSA, indicating that conformational changes associated with the protein were not restricted in a hard corona. The intrinsic behavior of the proteins driving the surface activity and compressibility of the complexes at an air–water interface was also observed at an air–lipid (RBC)–water interface. In this case the lipid monolayer acted as a barrier and reduced the interface concentration of bare nanoparticles. However, with a corona the nanoparticles penetrated into the monolayer and led to the formation of NP–HC–lipid ‘pillars’ that extended into air. Our results suggest that nanoparticle surface activity, and changes in surface activity due to corona formation, are insightful parameters to predicting nanoparticle–membrane interactions, complementing the conventional view that electrostatic forces are dominant.


 Please wait while we load your content...
Please wait while we load your content...