Interaction process between gaseous CH3I and NaCl particles: implication for iodine dispersion in the atmosphere†
Abstract
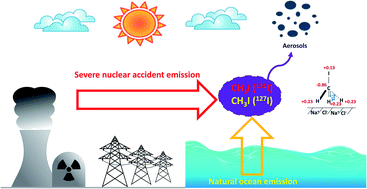
Gaseous iodomethane (CH3I) is naturally emitted into the atmosphere by biological activity in oceans and during severe accidents (SAs) in nuclear power plants. In this latter case, a part of radioactive iodine such as 131I may be released. Improving the knowledge of CH3I transport and reactivity in the atmosphere is important since they are strongly linked to first the cycle of ozone and second to the dispersion of radioactive CH3I with potential radiological consequences on both the environment and human health. Here, the interaction process of CH3I with NaCl as a surrogate of atmospheric aerosols was investigated under ambient air conditions by using Diffuse Reflectance Fourier Transform Spectroscopy (DRIFTS). The DRIFTS spectra of NaCl clearly evidenced CH3I adsorption on the NaCl particle surface. A part of CH3I ((1.68 ± 0.85) × 1014 molecule per mgNaCl) was found to be strongly bonded to NaCl since no desorption was observed. The CH3I adsorption on the NaCl surface presented a 1st order kinetics relative to its gas phase concentration. The uptake coefficient was determined to be in the order of 10−11. These results show a low probability of CH3I to be taken up by halide-containing aerosols. These data are crucial for completing the iodine atmospheric chemical scheme.


 Please wait while we load your content...
Please wait while we load your content...