Impacts of environmental levels of hydrogen peroxide and oxyanions on the redox activity of MnO2 particles†
Abstract
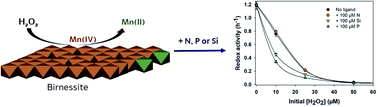
Despite the widespread presence of hydrogen peroxide (H2O2) in surface water and groundwater systems, little is known about the impact of environmental levels of H2O2 on the redox activity of minerals. Here we demonstrate that environmental concentrations of H2O2 can alter the reactivity of birnessite-type manganese oxide, an earth-abundant functional material, and decrease its oxidative activity in natural systems across a wide range of pH values (4–8). The H2O2-induced reductive dissolution generates Mn(II) that will re-bind to MnO2 surfaces, thereby affecting the surface charge of MnO2. Competition of Bisphenol A (BPA), used as a target compound here, and Mn(II) to interact with reactive surface sites may cause suppression of the oxidative ability of MnO2. This suppressive effect becomes more effective in the presence of oxyanions such as phosphate or silicate at concentrations comparable to those encountered in natural waters. Unlike nitrate, adsorption of phosphate or silicate onto birnessite increased in the presence of Mn(II) added or generated through H2O2-induced reduction of MnO2. This suggests that naturally occurring anions and H2O2 may have synergetic effects on the reactivity of birnessite-type manganese oxide at a range of environmentally relevant H2O2 amounts. As layered structure manganese oxides play a key role in the global carbon cycle as well as pollutant dynamics, the impact of environmental levels of hydrogen peroxide (H2O2/MnO2 molar ratio ≤ 0.3) should be considered in environmental fate and transport models.


 Please wait while we load your content...
Please wait while we load your content...