Temperature dependence of the gas-particle partitioning of selected VOCs†
Abstract
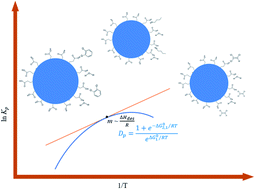
The gas-particle partitioning coefficients for volatile organic compounds (VOCs) are difficult to acquire because discriminating the small mass fraction of the VOCs in the aerosol particle relative to that in the gas phase is challenging. In this paper, we report the temperature dependence of the gas-particle partitioning coefficient (Kp) for n-butanol (n-BuOH) and trichloroethylene (TCE). Using the bench-scale system that we developed, we measured the Kp of surrogate VOCs, n-BuOH, and TCE onto inorganic (ammonium sulfate, Am Sulf) and organic (succinic acid, SA) aerosol particles at a fixed relative humidity (RH) of 35%. At this RH level and temperature range of 278.15–308.15 K, the ln Kp for TCE and n-BuOH partitioning on SA aerosol particles were −27.0 ± 0.70 to −27.9 ± 0.01 and −13.9 ± 0.03 to −17.4 ± 0.17. In contrast, the ln Kp for TCE and n-BuOH partitioning on Am Sulf aerosol particles ranged from −26.4 ± 0.70 to −27.4 ± 0.71 and −14.1 ± 0.03 to −17.1 ± 0.17, respectively. Results showed that TCE fitted well with the classic van't Hoff relationship. The enthalpy of desorption (ΔHdes) for TCE was constant over the temperature range of 278.15 K to 308.15 K, behaving similarly to 1,2-dichlorobenzene. At a similar temperature range, n-BuOH partitioning into both aerosol particles exhibited nonlinear temperature dependence. The minimum ratio of ΔHdes (Am Sulf:SA) for n-BuOH partitioning on each aerosol type was at ∼278.15 K. The magnitude of the entropy ΔSdes for all VOCs was <1 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...