Effects of environmental factors on nitrofurantoin photolysis in water and its acute toxicity assessment†
Abstract
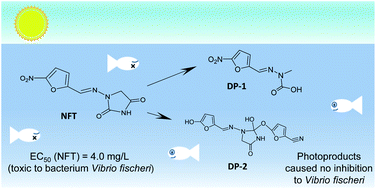
Pharmaceuticals have special attention of researchers over the world due to their possible effect on the environment and humans. This paper focuses on the photolysis of nitrofurantoin in different water matrices. Nitrofurantoin photodegradation has been indicated as a pseudo-first order photoreaction. The indirect photodegradation rate of nitrofurantoin (river water, k1 = 0.0088 min−1 and synthetic wastewater, k1 = 0.0154 min−1) was slower than its direct photolysis rate (ultrapure water, k1 = 0.0176 min−1). The highest value of quantum yield of nitrofurantoin photodegradation (ϕ = 0.2047) was observed at pH = 4, while at higher pH-values it decreased. Furthermore, the mechanism of nitrofurantoin photodegradation is proposed. Heterocyclic ring opening and further hydrolysis, nucleophilic aromatic photosubstitution and homolytic N–N bond cleavage are suggested as three main initial processes of nitrofurantoin photodegradation. Acute toxicity study of nitrofurantoin and its photoproducts with regard to luminescence inhibition of Vibrio fischeri showed that the toxic effect of nitrofurantoin (EC50 = 4.0 mg L−1) decreases by photolysis.


 Please wait while we load your content...
Please wait while we load your content...