High transference number enabled by sulfated zirconia superacid for lithium metal batteries with carbonate electrolytes†
Abstract
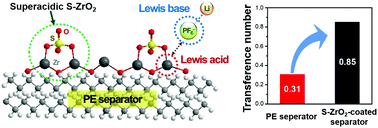
The prospect of increasing the energy density has promoted research on lithium metal batteries. Yet, avoiding the uncontrolled growth of lithium dendrites and the resulting interfacial instability to ensure the practical viability of the given battery technology remains a considerable challenge. Here, we report coating the separator with sulfated zirconia superacid to achieve a high lithium ion transference number of 0.85 and excellent cycle life when a full-cell paired with a LiNi0.82Co0.07Mn0.11O2 cathode was tested in a carbonate electrolyte under practical operating conditions. The exceptionally high transference number is attributed to strengthened binding of the PF6− anion of the lithium salt with the superacid. Furthermore, the presence of the superacid induces a mechanically stable solid–electrolyte-interphase (SEI) layer rich in LixPOyFz. This study demonstrates the beneficial effect of the superacid on emerging post-lithium-ion batteries by immobilizing the anion of the salt as well as modifying the SEI composition.


 Please wait while we load your content...
Please wait while we load your content...