A quasi-solid-state rechargeable cell with high energy and superior safety enabled by stable redox chemistry of Li2S in gel electrolyte†
Abstract
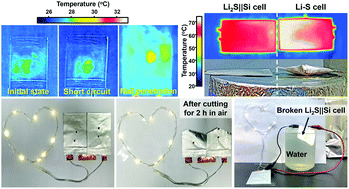
Recent years have witnessed a thriving pursuit of high-energy Li metal batteries for replacing the existing Li-ion batteries. However, the cell chemistry involving extremely reactive Li metal anodes in flammable organic liquid electrolytes raises serious problems in battery performance and safety. Herein, we report on achieving high energy and superior safety in a quasi-solid-state rechargeable cell design in terms of Li metal-free stable redox chemistry between a Li2S cathode and Si anode in gel polymer electrolyte with favorable interfacial properties, ionic conductivity and robustness. This chemistry ensures intrinsically high safety even under extreme conditions by eliminating the uncontrolled exothermic chain reactions in the cells. A nanospace-confined bifunctional electrocatalytic adsorber is designed to minimize the diffusion restriction of the Li2S cathode in the gel electrolyte, while the Si anode is strengthened by a multilevel hollow design. The obtained quasi-solid-state rechargeable Li2S||Si full cells deliver a high specific energy of up to 802 W h kgLi2S+Si−1 with high durability, low self-discharge, and good temperature adaptability from −20 to 60 °C. Meanwhile, the cells exhibit excellent safety against mechanical damage, overheating and short circuit in the air or water, offering high reliability for practical use.


 Please wait while we load your content...
Please wait while we load your content...