Impact of oxygen content on preferred localization of p- and n-type carriers in La0.5Sr0.5Fe1−xMnxO3−δ
Abstract
The oxygen content in La0.5Sr0.5Fe1−xMnxO3−δ, measured by coulometric titration in a wide range of oxygen partial pressure at various temperatures, was used for defect chemistry analysis. The obtained data were well approximated by a model assuming defect formation in La0.5Sr0.5Fe1−xMnxO3−δvia Fe3+ and Mn3+ oxidation reactions and charge disproportionation on Fe3+ and Mn3+ ions. The partial molar enthalpy and entropy of oxygen in La0.5Sr0.5Fe1−xMnxO3−δ obtained by statistical thermodynamic calculations were found to be in satisfactory agreement with those obtained using the Gibbs–Helmholtz equations, thus further confirming the adequacy of the model. The impact of manganese substitution on defect equilibrium in La0.5Sr0.5Fe1−xMnxO3−δ was shown to be attributed to a lower enthalpy of Mn3+ oxidation reaction (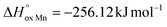 vs.
vs.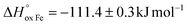 for the oxidation of Fe3+) and the charge disproportionation reaction on Mn3+ (
for the oxidation of Fe3+) and the charge disproportionation reaction on Mn3+ (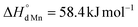 vs.
vs.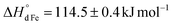 for that on Fe3+). The former makes Mn4+ ions more resistant to reduction than Fe4+. The latter favors the presence of Mn2+, Mn3+, and Mn4+ ions in oxides in comparable concentrations. The distribution of charge carriers over iron and manganese ions was determined as a function of oxygen content in La0.5Sr0.5Fe1−xMnxO3−δ.
for that on Fe3+). The former makes Mn4+ ions more resistant to reduction than Fe4+. The latter favors the presence of Mn2+, Mn3+, and Mn4+ ions in oxides in comparable concentrations. The distribution of charge carriers over iron and manganese ions was determined as a function of oxygen content in La0.5Sr0.5Fe1−xMnxO3−δ.



 Please wait while we load your content...
Please wait while we load your content...