Activation of sodium borohydride via carbonyl reduction for the synthesis of amine- and phosphine-boranes†
Abstract
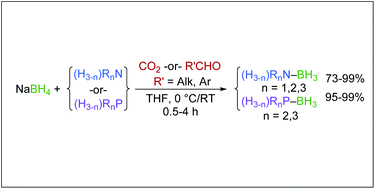
A highly versatile synthesis of amine-boranes via carbonyl reduction by sodium borohydride is described. Unlike the prior bicarbonate-mediated protocol, which proceeds via a salt metathesis reaction, the carbon dioxide-mediated synthesis proceeds via reduction to a monoformatoborohydride intermediate. This has been verified by spectroscopic analysis, and by using aldehydes and ketones as the carbonyl source for the activation of sodium borohydride. This process has been used to produce borane complexes with 1°-, 2°-, and 3°-amines, including those with borane reactive functionalities, heteroarylamines, and a series of phosphines.

 Please wait while we load your content...
Please wait while we load your content...