Organometallic chemistry and application of palladacycles in asymmetric hydrophosphination reactions†
Abstract
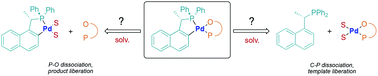
A number of palladacycles containing chiral chelating auxiliaries have been utilized as efficient catalysts for asymmetric hydrophosphination reactions. In all cases, the chiral auxiliaries remained coordinated to the palladium centres throughout the course of the reactions. Despite the presence of a large quantity of powerful tertiary phosphines, which are known to be strong metal ion sequesters, the expected catalyst poisoning was rarely observed in these palladacycle catalyzed processes. This review highlights the unique stereoelectronic features and the important organometallic chemistry of palladacycle catalysts which are essential to their synthetic operations.
- This article is part of the themed collection: 2021 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...