The protonation state governs the coordination of phosphinoferrocene guanidines†
Abstract
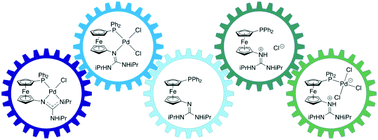
Compared to phosphines with guanidinium tags, studied as polar ligands for aqueous catalysis, their counterparts bearing guanidine substituents received only limited attention. This contribution focuses on the coordination of phosphinoferrocene guanidine Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NHiPr)2 (1iPr, fc = ferrocene-1,1′-diyl) as a hybrid, P,N-donor ligand to Group 10 metals. In its native state, 1iPr coordinated as a P,N-chelating ligand, affording [M(X)(Y)(1iPr-κ2P,N)] (M/X/Y = Pd/Cl/Cl, Pd/Br/4-C6H4CN, Pt/Cl/Cl; the corresponding Ni(II) complex was not isolated). While [PdCl2(1iPr-κ2P,N)] converted into [PdCl(1iPr-κ3Fe,P,N)]+ species with Fe–Pd interaction, upon chloride removal, the analogous Pt(II) complex dimerised into [Pt2(μ-Cl)2(1iPr-κ2P,N)2]2+. Deprotonation of [PdCl2(1iPr-κ2P,N)] produced a unique, doubly chelating phosphinoguanidinate complex [PdCl{(1iPr–H)-κ3P,N,N′}], which was smoothly converted into [Pd(MeCN){(1iPr–H)-κ3P,N,N′}][SbF6]. The latter, a convenient starting material for substitution reactions, was used to prepare either [Pd(L){(1iPr–H)-κ3P,N,N′}][SbF6] (L = 4-(dimethylamino)pyridine and 2-phenylpyridine), by simple substitution, or the hydroxide and acetylacetonate (acac) complexes, [Pd2(μ-OH)2(1iPr-κ2P,N)2][SbF6]2 and [Pd(acac)(1iPr-κ2P,N)][SbF6], by substitution with concomitant proton transfer. In contrast, protonation of the guanidine moiety prevented its coordination, as shown in reactions of the salts (1iPrH)Cl and (1iPrH)[SbF6]. Depending on the metal-to-ligand ratio, adding (1iPrH)[SbF6] to [PdCl2(MeCN)2] produced [Pd2Cl2(μ-Cl)2(1iPrH-κP)2][SbF6]2 or [PdCl2(1iPrH-κP)2][SbF6]2. Analogous reactions involving (1iPrH)Cl were more complicated due to competing coordination of the chloride anion, leading to (in addition to other compounds) the zwitterionic complex [PdCl3(1iPrH-κP)], which was alternatively obtained by selective protonation of [PdCl2(1iPr-κ2P,N)] with HCl. Apparently, the protonation state of the guanidine moiety controls the coordination behaviour of phosphinoferrocene guanidines.
C(NHiPr)2 (1iPr, fc = ferrocene-1,1′-diyl) as a hybrid, P,N-donor ligand to Group 10 metals. In its native state, 1iPr coordinated as a P,N-chelating ligand, affording [M(X)(Y)(1iPr-κ2P,N)] (M/X/Y = Pd/Cl/Cl, Pd/Br/4-C6H4CN, Pt/Cl/Cl; the corresponding Ni(II) complex was not isolated). While [PdCl2(1iPr-κ2P,N)] converted into [PdCl(1iPr-κ3Fe,P,N)]+ species with Fe–Pd interaction, upon chloride removal, the analogous Pt(II) complex dimerised into [Pt2(μ-Cl)2(1iPr-κ2P,N)2]2+. Deprotonation of [PdCl2(1iPr-κ2P,N)] produced a unique, doubly chelating phosphinoguanidinate complex [PdCl{(1iPr–H)-κ3P,N,N′}], which was smoothly converted into [Pd(MeCN){(1iPr–H)-κ3P,N,N′}][SbF6]. The latter, a convenient starting material for substitution reactions, was used to prepare either [Pd(L){(1iPr–H)-κ3P,N,N′}][SbF6] (L = 4-(dimethylamino)pyridine and 2-phenylpyridine), by simple substitution, or the hydroxide and acetylacetonate (acac) complexes, [Pd2(μ-OH)2(1iPr-κ2P,N)2][SbF6]2 and [Pd(acac)(1iPr-κ2P,N)][SbF6], by substitution with concomitant proton transfer. In contrast, protonation of the guanidine moiety prevented its coordination, as shown in reactions of the salts (1iPrH)Cl and (1iPrH)[SbF6]. Depending on the metal-to-ligand ratio, adding (1iPrH)[SbF6] to [PdCl2(MeCN)2] produced [Pd2Cl2(μ-Cl)2(1iPrH-κP)2][SbF6]2 or [PdCl2(1iPrH-κP)2][SbF6]2. Analogous reactions involving (1iPrH)Cl were more complicated due to competing coordination of the chloride anion, leading to (in addition to other compounds) the zwitterionic complex [PdCl3(1iPrH-κP)], which was alternatively obtained by selective protonation of [PdCl2(1iPr-κ2P,N)] with HCl. Apparently, the protonation state of the guanidine moiety controls the coordination behaviour of phosphinoferrocene guanidines.


 Please wait while we load your content...
Please wait while we load your content...