Influence of temperature on the equilibria of oxidovanadium(iv) complexes in solution†
Abstract
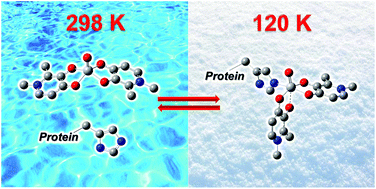
The equilibria in the solution of three different oxidovanadium(IV) complexes, VO(dhp)2 (dhp = 1,2-dimethyl-3-hydroxy-4(1H)-pyridinonato), VO(ma)2 (ma = maltolato) and VO(pic)2(H2O) (pic = picolinato), were examined in the temperature range of 120–352 K through a combination of instrumental (EPR spectroscopy) and computational techniques (DFT methods). The results revealed that a general equilibrium exists: VOL2 + H2O ⇄ cis-VOL2(H2O) ⇄ trans-VOL2(H2O), where cis and trans refer to the relative position of H2O and the oxido ligand. The equilibrium is more or less shifted to the right depending on the ligand, the temperature, the ionic strength and the coordinating properties of the solvent. With VO(dhp)2, only the square pyramidal species exists at 298 K in aqueous solution, while at 120 K the cis- and trans-VO(dhp)2(H2O) species are also present. The complex of maltol exists almost exclusively in the form cis-VO(ma)2(H2O) in aqueous solution at 298 K, while the trans species can be revealed only at higher temperatures, where the EPR linewidth significantly decreases. The equilibria involving 1-methylimidazole (MeIm), a model for the side chain His coordination, are also influenced by temperature, with its coordination being favored by decreasing the temperature. The implications of these results in the study of the (vanadium complex)–protein systems are discussed and the interaction with myoglobin (Mb) is examined as a representative example.


 Please wait while we load your content...
Please wait while we load your content...