A chromotropic PtIIPdIICoII coordination polymer with dual electrocatalytic activity for water reduction and oxidation†
Abstract
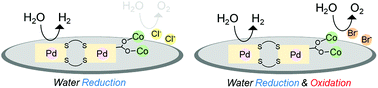
Here, we present a heterometallic coordination polymer that exhibits heterogeneous electrocatalytic activities for both water reduction and water oxidation. Treatment of the PtII2PdII2 tetranuclear complex [Pd2{Pt(NH3)2(D-pen)2}2] ([1]; D-H2pen = D-penicillamine) with CoX2 (X = Cl, Br) provided (PtII2PdII2CoII2)n coordination polymers [Co2(H2O)6(1)]X4 ([2]X4), in which the PtII2PdII2 units of [1] are linked by [Co2(μ-H2O)(H2O)5]4+ moieties in a 3D network structure. [2]X4 showed a colour change from orange to dark green upon dehydration, reflecting the geometrical conversion of the CoII centres in [Co2(μ-H2O)(H2O)5]4+ from an octahedron to a tetrahedron by the removal of aqua ligands. While both [2]Cl4 and [2]Br4 electrochemically catalysed water reduction to H2 in the solid state due to the presence of PdII active centres, water oxidation to O2 was catalysed only by [2]Br4, which is ascribed to the presence of Br− ions that mediate the catalytic reactions that occurred at CoII active centres.


 Please wait while we load your content...
Please wait while we load your content...