Abstract
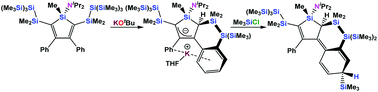
Reaction of a 3,4-diphenylsilole with two neopentasilanyl groups attached to the 2- and 5-positions with one equivalent of KOtBu did not result in the expected silanide formation but yielded a silole allylic anion instead. The initially formed silanide added to a neighboring phenyl group, which then transfers a proton to the 2-position of the silole ring.


 Please wait while we load your content...
Please wait while we load your content...