Crystal structure of silver pentazolates AgN5 and AgN6†
Abstract
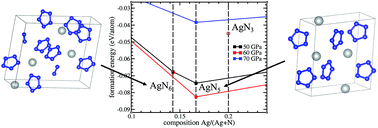
Silver pentazolate, a high energy density compound containing the cyclo-N5− anion, has recently been synthesized under ambient conditions. However, due to high sensitivity to irradiation, its crystal structure has not been determined. In this work, silver–nitrogen crystalline compounds under ambient conditions and at high pressures, up to 100 GPa, are predicted and characterized by performing first-principles evolutionary crystal structure searching with variable stoichiometry. It is found that newly discovered AgN5 and AgN6 are the only thermodynamically stable silver–nitrogen compounds at pressures between 42 and 80 GPa. In contrast to AgN5, the pentazolate AgN6 compound contains N2 diatomic molecules in addition to cyclo-N5−. These AgN5 and AgN6 crystals are metastable under ambient conditions with positive formation enthalpies of 54.95 kJ mol−1 and 46.24 kJ mol−1, respectively. The underlying cause of the stability of cyclo-N5− silver pentazolates is the enhanced aromaticity enabled by the charge transfer from silver atoms to nitrogen rings. To aid in the experimental identification of these materials, calculated Raman spectra are reported at ambient pressure: the frequencies of N5− vibrational modes of AgN5 are in good agreement with those measured in the experiment.


 Please wait while we load your content...
Please wait while we load your content...