Synthesis, crystal structures, anticancer activities and molecular docking studies of novel thiazolidinone Cu(ii) and Fe(iii) complexes targeting lysosomes: special emphasis on their binding to DNA/BSA†
Abstract
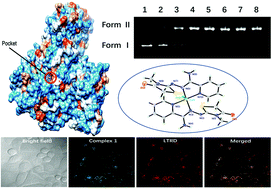
Novel [CuL2Cl]Cl·H2O (1) and [FeL2Cl2]Cl·MeOH·CHCl3·H2O (2) complexes of (Z)-N′-((E)-3-methyl-4-oxothiazolidin-2-ylidene)picolinohydrazonamide (L) as antitumor agents were designed and synthesized in order to explore DNA and serum albumin interaction. X-ray diffraction revealed that both 1 and 2 were a triclinic crystal system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, which consisted of a positive electric main unit, a negative chloride ion and some solvent molecules. The complexes with DNA and bovine serum albumin (BSA) were studied by fluorescence and electronic absorption spectrometry. The results indicated that there was moderate intercalative binding mode between the complexes and DNA with Kapp values of 2.40 × 105 M−1 (1) and 6.49 × 105 M−1 (2). Agarose gel electrophoresis experiment showed that both 1 and 2 exhibited obvious DNA cleavage activity via an oxidative DNA damage pathway, and the cleavage activities of 1 were stronger than those of 2. Cytotoxicity assay showed that 1 had a more effective antitumor activity than 2. The two complexes were bound to BSA by a high affinity and quenched the fluorescence of BSA through a static mechanism. The thermodynamic parameters suggested that hydrophobic interactions played a key role in the binding process. The binding energy xpscore of 1 and 2 were −10.529 kcal mol−1 and −10.826 kcal mol−1 by docking studies, and this suggested that the binding process was spontaneous. Complex 1 displayed a lysosome-specific targeting behavior with a Pearson coefficient value of 0.82 by confocal laser scanning microscopy (CLSM), and accumulated in the lysosomes, followed by the disruption of lysosomal integrity.
space group, which consisted of a positive electric main unit, a negative chloride ion and some solvent molecules. The complexes with DNA and bovine serum albumin (BSA) were studied by fluorescence and electronic absorption spectrometry. The results indicated that there was moderate intercalative binding mode between the complexes and DNA with Kapp values of 2.40 × 105 M−1 (1) and 6.49 × 105 M−1 (2). Agarose gel electrophoresis experiment showed that both 1 and 2 exhibited obvious DNA cleavage activity via an oxidative DNA damage pathway, and the cleavage activities of 1 were stronger than those of 2. Cytotoxicity assay showed that 1 had a more effective antitumor activity than 2. The two complexes were bound to BSA by a high affinity and quenched the fluorescence of BSA through a static mechanism. The thermodynamic parameters suggested that hydrophobic interactions played a key role in the binding process. The binding energy xpscore of 1 and 2 were −10.529 kcal mol−1 and −10.826 kcal mol−1 by docking studies, and this suggested that the binding process was spontaneous. Complex 1 displayed a lysosome-specific targeting behavior with a Pearson coefficient value of 0.82 by confocal laser scanning microscopy (CLSM), and accumulated in the lysosomes, followed by the disruption of lysosomal integrity.


 Please wait while we load your content...
Please wait while we load your content...