Pseudotetrahedral copper(ii)-complexes with enantiopure (R or S)-2-(((aryl)ethylimino)ethyl)phenolate Schiff base ligands†
Abstract
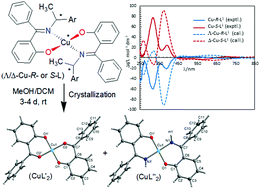
Condensation of 2-hydroxy-benzophenone (HL′) with (R or S)-(Ar)ethylamine yields the enantiopure Schiff bases (S or R)-2-((E)-1-(1-(Ar)ethylimino)ethyl)phenol {Ar = C6H5 (S- or R-HL1), p-CH3OC6H4 (S- or R-HL2)}. These Schiff bases react with copper(II) acetate under reflux to give green microcrystals of bis[(R or S)-2-((E)-1-(1-(Ar)ethylimino)ethyl)phenolato-κ2N,O]-Λ/Δ-copper(II), {Ar = C6H5 (Λ/Δ-Cu-R- or S-L1), p-CH3OC6H4 (Λ/Δ-Cu-R- or S-L2)} with induction of Λ/Δ-chirality at-metal. The presence of Schiff base ligands in the paramagnetic green microcrystals is confirmed by decomplexation reaction with NaCN via reduction of Cu(II) to Cu(I) in DMSO-d6 solution. Crystallization attempts of the green microcrystalline Schiff-base Cu complexes provide deep-green block-shaped crystals of an about equal admixture of bis[2-oxo-benzophenonato-κ2O,O′]-copper(II), (CuL′2) and bis[2-(imino(phenyl)methyl)phenolato-κ2N,O]copper(II), (CuL′′2) via in situ hydrolysis of the coordinated Schiff base ligands back to 2-hydroxy-benzophenone (HL′) and to 2-(imino(phenyl)methyl)phenol (HL′′), which in-turn bind with the copper(II) ion. Powder X-ray diffraction (PXRD) patterns of R-HL1 and Cu-R-L1 allowed their structure determinations using the program Expo-2014 followed by Rietveld refinement. The Cu structures refined to four-coordinated Λ/Δ-copper(II)-complexes by the two phenolate-oxygen and two imine-nitrogen atoms from two Schiff base ligands in a pseudotetrahedral geometry. DFT optimized structures (at gas-phase) reveal the Δ-Cu-S-L1 or Λ-Cu-R-L1 diastereomer as slightly more stable than the corresponding Λ-Cu-S-L1 or Δ-Cu-R-L1 by ca. 7.60 kcal mol−1, resulting from diastereoselectively induced Λ vs. Δ-chirality at-metal. Electronic circular dichroism (ECD) spectra display mirror-image relationships and comparisons of experimental and simulated ECD spectra by TDDFT suggest an excess of the Δ-Cu-S-L1 or Λ-Cu-R-L1 diastereomer in solution. The cyclic voltammograms demonstrate two one electron charge transfer processes for Cu2+/Cu+ and Cu+/Cu0 couples in acetonitrile, respectively.
- This article is part of the themed collection: Celebrating our Golden Authors


 Please wait while we load your content...
Please wait while we load your content...