Hydrogenation of CO2 to methanol catalyzed by a manganese pincer complex: insights into the mechanism and solvent effect†
Abstract
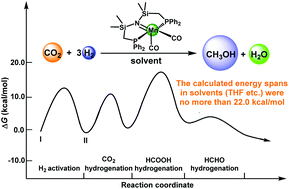
Herein, density functional theory (DFT) calculations were employed to explore the reaction mechanism of three cascade cycles for the hydrogenation of carbon dioxide to methanol (CO2 + 3H2 → CH3OH + H2O) catalyzed by a manganese pincer complex [Mn(Ph2PCH2SiMe2)2N(CO)2]. The three cascade cycles involve: the hydrogenation of CO2 to formic acid, the hydrogenation of formic acid to methanediol and the decomposition of methanediol to formaldehyde and water, and the hydrogenation of formaldehyde to methanol. The calculated results demonstrate that hydrogen activation is the rate-determining step of each catalytic cycle under solvent-free conditions, and the energy span of the whole reaction is 27.1 kcal mol−1. Furthermore, the solvent was found to be of importance in this reaction. In three different solvents, the rate-determining steps of this reaction are all the hydrogen transfer step of the formic acid hydrogenation stage, and the corresponding energy spans in water, toluene and THF solvents are 21.3, 20.8 and 20.4 kcal mol−1, respectively. Such a low energy span implies that this manganese complex could be a promising catalyst for the efficient conversion of CO2 and H2 to methanol at temperatures below 100–150 °C.


 Please wait while we load your content...
Please wait while we load your content...