Effect of cytosine–Ag+–cytosine base pairing on the redox potential of the Ag+/Ag couple and the chemical reduction of Ag+ to Ag by tetrathiafulvalene†
Abstract
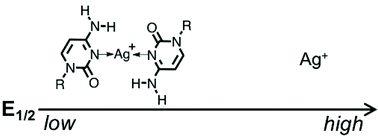
The redox properties of metallo-base pairs remain to be elucidated. Herein, we report the detailed 1H/13C/109Ag NMR spectroscopic and cyclic voltammetric characterisation of the [Ag(cytidine)2]+ complex as isolated cytosine–Ag+–cytosine (C–Ag+–C) base pairs. We also performed comparative studies between cytidine/Ag+ and other nucleoside/Ag+ systems by using cyclic voltammetry measurements. In addition, to evaluate the effect of [Ag(cytidine)2]+ formation on the chemical reduction of Ag+ to Ag, we utilised the redox reaction between Ag+ and tetrathiafulvalene (TTF). We found that Ag+-mediated base pairing lowers the redox potential of the Ag+/Ag couple. In addition, C–Ag+–C base pairing makes it more difficult to reduce captured Ag+ ions than in other nucleoside/Ag+ systems. Remarkably, the cytidine/Ag+ system can be utilised to control the redox potential of the Ag+/Ag couple in DMSO. This feature of the cytidine/Ag+ system may be exploited for Ag nanoparticle synthesis by using the redox reaction between Ag+ and TTF.


 Please wait while we load your content...
Please wait while we load your content...