Crystal transformation in Mn(ii) metal–organic frameworks based on a one-dimensional chain precursor†
Abstract
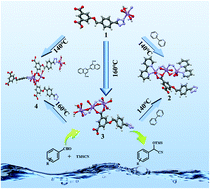
The solvothermal reaction of Mn(II) salts and 5-((4′-(tetrazol-5′′-yl)benzyl)oxy)isophthalic acid (H3L) affords an Mn(II) based coordination polymer Mn(H2L)2(H2O)2 (1), which possesses a one-dimensional (1D) chain structure. Using 1 as the precursor, three Mn(II) metal–organic frameworks, Mn3L2(2,2′-bpy)2·5H2O (2), Mn3L2(H2O)4 (3), and Mn4L2(HL)(H2O)5·0.5H2O (4), with three-dimensional (3D) networks can be obtained by different strategies of crystal transformation. Upon introduction of 2,2′-bipyridine (2,2′-bpy) as the ligand and 2,2′-biquinoline-4,4′-dicarboxylic acid as the structural-directing agent, 1 undergoes irreversible crystal transformation into 2 and 3, respectively, and 1 can be transformed into 4 by increasing the reaction temperature. Interestingly, the irreversible structural transformation of 3 into 2 can be carried out by adding a 2,2′-bpy ligand. Notably, after the removal of coordinated water molecules, 1 and 3 exhibit good catalytic performance for the cyanosilylation reaction even at 0 °C.


 Please wait while we load your content...
Please wait while we load your content...